Matter
is made up of very tiny units called atoms. Each different type of atom is the
building block of a different chemical element. Presently, the International
Union of Pure and Applied Chemistry (IUPAC) recognizes 112 elements, and all
matter is made up of just these types! The known elements range from common
substances, such as carbon, iron, and silver, to uncommon ones, such as
lutetium and thulium. About 90 of the elements can be obtained from natural
sources. The remainder do not occur naturally and have been created only in
laboratories. On the inside front cover you will find a complete listing of the
elements and also a special tabular arrangement of the elements known as the
periodic table. The periodic table is the chemist s directory of the elements.
We will describe it in Chapter 2and use it throughout most of the text.
Chemical
compounds are substances comprising atoms of two or more elements joined
together. Scientists have identified millions of different chemical compounds.
In some cases, we can isolate a molecule of a compound. A molecule is the
smallest entity having the same proportions of the constituent atoms as does
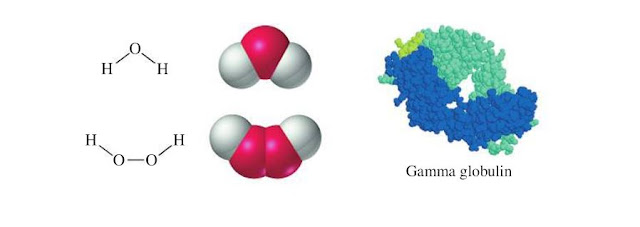
the compound as a whole. A molecule of water consists of three atoms: two
hydrogen atoms joined to a single oxygen atom. A molecule of hydrogen peroxide
has two hydrogen atoms and two oxygen atoms; the two oxygen atoms are joined
together and one hydrogen atom is attached to each oxygen atom. By contrast, a
molecule of the blood protein gamma globulin is made up of 19,996 atoms, but
they are of just four types: carbon, hydrogen, oxygen, and nitrogen.
The
composition and properties of an element or a compound are uniform throughout a
given sample and from one sample to another. Elements and compounds are called
substances. (In the chemical sense, the term substance should be used only for
elements and compounds.) A mixture of substances can vary in composition and
properties from one sample to another. One that is uniform in composition and
properties throughout is said to be a homogeneous mixture or a solution. A
given solution of sucrose (cane sugar) in water is uniformly sweet throughout
the solution, but the sweetness of another sucrose solution may be rather
different if the sugar and water are present in different proportions. Ordinary
air is a homogeneous mixture of several gases, principally the elements
nitrogen and oxygen. Seawater is a solution of the compounds water, sodium
chloride (salt), and a host of others. Gasoline is a homogeneous mixture or
solution of dozens of compounds.
 |
| Is it homogeneous or heterogeneous? When viewed through a microscope, homogenized milk is seen to consist of globules of fat dispersed in a watery medium. Homogenized milk is a heterogeneous mixture. |
In
heterogeneous mixtures sand and water, for example the components separate into
distinct regions. Thus, the composition and physical properties vary from one
part of the mixture to another. Salad dressing, a slab of concrete, and the leaf
of a plant are all heterogeneous. It is usually easy to distinguish
heterogeneous from homogeneous mixtures. A scheme for classifying matter into
elements and compounds and homogeneous and heterogeneous mixtures is summarized
in Figure below.
Separating
Mixtures
A
mixture can be separated into its components by appropriate physical means.
Consider again the heterogeneous mixture of sand in water. When we pour this
mixture into a funnel lined with porous filter paper, the water passes through
and sand is retained on the paper. This process of separating a solid from the
liquid in which it is suspended is called filtration (Figure). You will
probably use this procedure in the laboratory. Conversely, we cannot separate a
homogeneous mixture (solution) of copper (II) sulfate in water by filtration
because all components pass through the paper. We can, however, boil the
solution of copper (II) sulfate and water. In the process of distillation, a
pure liquid is condensed from the vapor given off by a boiling solution. When
all the water has been removed by boiling a solution of copper (II) sulfate in
water, solid copper (II) sulfate remains behind.
Another
method of separation available to modern chemists depends on the differing
abilities of compounds to adhere to the surfaces of various solid substances,
such as paper and starch. The technique of chromatography relies on this
principle. The dramatic results that can be obtained with chromatography are
illustrated by the separation of ink on a filter paper.
Decomposing
Compounds
A chemical
compound retains its identity during physical changes, but it can be decomposed
into its constituent elements by chemical changes. The decomposition of
compounds into their constituent elements is a more difficult matter than the mere
physical separation of mixtures. The extraction of iron from iron oxide ores
requires a blast furnace. The industrial production of pure magnesium from
magnesium chloride requires electricity. It is generally easier to convert a
compound into other compounds by a chemical reaction than it is to separate a
compound into its constituent elements. For example, when heated, ammonium
dichromate decomposes into the substances chromium (III) oxide, nitrogen, and
water. This reaction, once used in movies to simulate a volcano, is illustrated
in Figure below.
 |
| A chemical change: decomposition of ammonium dichromate |
States
of Matter
Matter
is generally found in one of three states: solid, liquid, or gas. In a solid,
atoms or molecules are in close contact, sometimes in a highly organized
arrangement called a crystal. A solid has a definite shape. In a liquid, the
atoms or molecules are usually separated by somewhat greater distances than in
a solid. Movement of these atoms or molecules gives a liquid it’s most
distinctive property the ability to flow, covering the bottom and assuming the
shape of its container. In a gas, distances between atoms or molecules are much
greater than in a liquid. A gas always expands to fill its container. Depending
on conditions, a substance may exist in only one state of matter, or it may be
present in two or three states. Thus, as the ice in a small pond begins to melt
in the spring, water is in two states: solid and liquid (actually, three states
if we also consider water vapor in the air above the pond). The three states of
water are illustrated at two levels in Figure.
The
macroscopic level refers to how we perceive matter with our eyes, through the
outward appearance of objects. The microscopic level describes matter as
chemists conceive of it in terms of atoms and molecules and their behavior. In
this text, we will describe many macroscopic, observable properties of matter,
but to explain these properties, we will often shift our view to the atomic or
molecular level the microscopic level.



No comments:
Post a Comment